Artificial Intelligence (AI) in drug development fundamentally accelerates discovery by utilizing machine learning, deep learning, and generative models to process biological data at speeds vastly exceeding human capability. AI speeds discovery by identifying novel disease targets, designing perfectly matched therapeutic molecules from scratch, predicting clinical trial outcomes, and simulating drug toxicity before physical testing. By shifting the heavy lifting from physical laboratories to computational models (“in silico” experiments), AI drastically reduces the traditional decade-long timeline and multi-billion dollar cost of bringing a new drug to market, improving the success rate of finding viable treatments for complex diseases.
Introduction: The Billion-Dollar Bottleneck
For decades, the pharmaceutical industry has operated under a model characterized by high risk, immense cost, and agonizingly slow progress. The industry standard is daunting: it typically takes 10 to 15 years and an average investment of over $2.5 billion to bring a single new drug to market.
Worse still is the failure rate. Approximately 90% of drug candidates that enter clinical trials fail. They fail because they don’t work as intended, they are toxic, or they are no better than existing treatments. This phenomenon of exponentially increasing costs despite technological improvements is often jokingly referred to as “Eroom’s Law” (Moore’s Law spelled backward).
We are facing a biological data explosion. We have mapped the human genome and have access to vast repositories of chemical information. Yet, humans alone cannot connect the dots within terabytes of complex, unstructured data. This is where Artificial Intelligence steps in, not merely as a tool for efficiency, but as a fundamental paradigm shift in how we understand biology and treat disease.
The Human Touch in a Digital World
It is crucial to remember that AI is not replacing scientists. Instead, it is augmenting human intelligence. It acts as a tireless research assistant, freeing up medicinal chemists and biologists to focus on creative problem-solving and critical decision-making rather than brute-force data crunching.
The AI Toolkit: A Brief Overview
Before diving into the how, it is helpful to understand the what. The specific types of AI used in pharma are specialized:
- Machine Learning (ML): Algorithms that learn patterns from existing databases (e.g., knowing which chemical structures have historically been toxic).
- Deep Learning (DL): A more complex subset of ML inspired by the human brain’s neural networks. It is exceptional at recognizing intricate patterns in unstructured data, such as interpreting microscopic images of cells or analyzing genetic sequences.
- Natural Language Processing (NLP): AI that can “read” and understand human language. In pharma, NLP mines millions of scientific papers, patents, and clinical notes to find hidden connections between genes and diseases.
- Generative AI: The newest frontier. Instead of just analyzing existing data, these models can create new data. In drug discovery, this means designing entirely new molecule structures that have never existed before.
The Four Stages of AI-Driven Acceleration
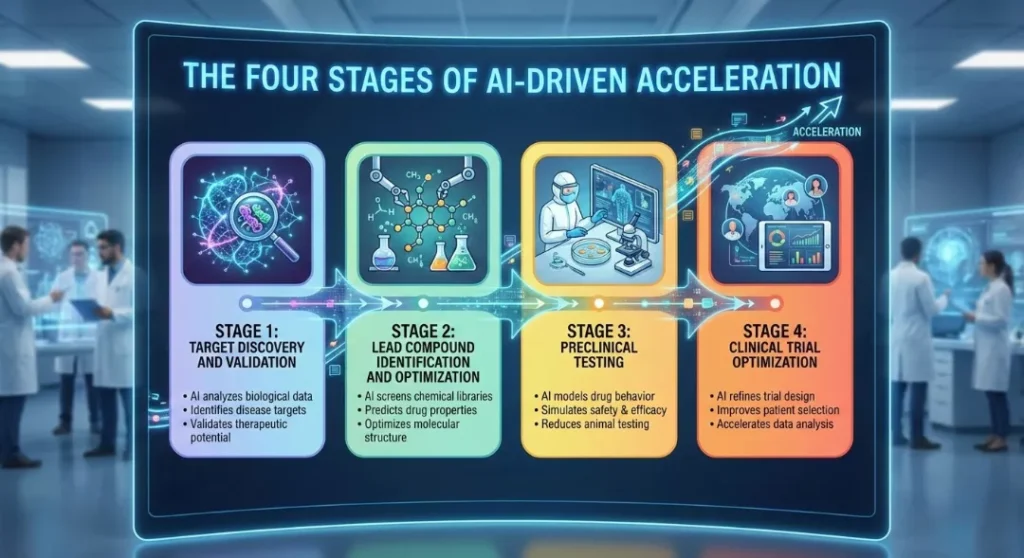
AI is applying pressure to every point in the drug discovery pipeline. Here is how it transforms the four critical stages.
Stage 1: Target Discovery and Validation (Finding the Lock)
Before you can make a key (a drug), you must identify the correct lock (a biological target, usually a protein linked to a disease). Traditionally, this involved years of academic research and guesswork.
How AI speeds it up:
AI algorithms, particularly Deep Learning and NLP, scour genomic data, patient records, and scientific literature. They identify obscure correlations—perhaps a specific gene mutation appears frequently in patients with a certain cancer but is absent in healthy individuals. AI can predict which proteins are “druggable” (capable of being affected by a medication) and validate that hitting this target will actually impact the disease.
Stage 2: Lead Compound Identification and Optimization (Designing the Key)
Once the target is found, the hunt begins for a “lead compound”—a molecule that interacts with the target correctly. The chemical universe contains an estimated $10^{60}$ potential drug-like molecules. Physically synthesizing and testing even a fraction of these is impossible.
How AI speeds it up (The Revolution of Generative AI):
This is perhaps the most dramatic area of acceleration.
- Virtual Screening: Instead of testing chemicals in a wet lab, AI performs “virtual high-throughput screening,” checking billions of existing molecules against a digital model of the target protein in days.
- De Novo Design: Generative AI models (similar to those that create art) are trained on chemistry rules. They can “imagine” and design brand-new molecules specifically tailored to fit the target protein perfectly, while simultaneously optimizing for properties like solubility and absorption.
Stage 3: Preclinical Testing (In Silico Safety)
Before a drug touches a human, it must be proven reasonably safe in cell cultures and animal models. This stage is notorious for late-stage failures where a promising drug turns out to be toxic to the liver or heart.
How AI speeds it up:
AI models act as “virtual organisms.” By training on vast databases of known toxic compounds and their effects on biology, these models can predict the toxicity of a new, untested molecule with high accuracy. This allows researchers to “fail early and fail cheap” in the computer, rather than waiting for expensive animal studies.
Stage 4: Clinical Trial Optimization (The Human Element)
Clinical trials are the most expensive and lengthy part of development. Finding the right patients and retaining them is a massive logistical hurdle.
How AI speeds it up:
AI analyzes electronic health records (EHRs) to identify ideal patient populations for trials, ensuring diversity and specific genetic criteria are met faster. Furthermore, AI is being used to create “synthetic control arms”—using real-world historical patient data to model a placebo group, potentially reducing the number of actual patients needed for a trial.
The AlphaFold Moment: A Game Changer
No discussion of AI in biology is complete without mentioning AlphaFold. Developed by DeepMind (a Google subsidiary), AlphaFold solved a 50-year-old grand challenge in biology: the “protein folding problem.”
Proteins are long chains of amino acids that fold into complex 3D shapes. That shape determines what the protein does. Knowing the shape is essential for designing a drug that fits it. Before AI, determining a single protein structure via X-ray crystallography could take a PhD student their entire academic career.
AlphaFold successfully predicted the 3D structures of nearly every known protein—over 200 million of them—and made the data freely available. This is the equivalent of suddenly being given a detailed map of an uncharted continent. It provides drug hunters with immediate access to the structures of thousands of potential disease targets that were previously mysteries.
Comparison Table: Traditional vs. AI Approaches
The following table summarizes the key differences in methodology and efficiency.
| Feature | Traditional Drug Discovery | AI-Augmented Drug Discovery |
| Data Analysis | Manual interpretation by scientists; limited scope. | Machine learning analyzes terabytes of omics data and literature instantly. |
| Finding Molecules | Screening physical libraries of existing chemicals. | Generative AI designs novel molecules; virtual screening of billions. |
| Testing (Preclinical) | Relies heavily on expensive wet labs and animal models. | “In silico” (computer) modeling predicts efficacy and toxicity early. |
| Timeline to Trials | 4–6 Years | 1–3 Years |
| Cost Efficiency | High rates of late-stage failure waste billions. | “Fail fast” approach in computers saves significant resources. |
Challenges, Ethics, and the Road Ahead
While the potential is immense, AI is not a magic wand. There are significant hurdles to overcome to fully realize its potential in saving lives.
The “Garbage In, Garbage Out” Problem
AI is a reflection of its training data. In the biological sciences, the quality of that reflection determines whether a drug saves lives or fails in clinical trials.
- Data Fragmentation and Silos: Biological data is often trapped within the “walled gardens” of private pharmaceutical companies or disparate academic institutions. Without a unified, open-access approach to data sharing, AI models lack the diverse datasets needed to identify universal biological patterns.
- The Representation Gap: Historical medical research has a significant bias toward European phenotypes. If training data lacks genetic diversity, the resulting treatments may be less effective—or even unsafe—for underrepresented populations, further widening the global health equity gap.
- Noise and Complexity: Unlike digital data, biological systems are inherently “noisy.” Laboratory conditions, sample degradation, and human error in recording data can lead to inaccuracies that AI might mistakenly interpret as meaningful signals.
The “Black Box” Phenomenon
In healthcare, “trust me” isn’t enough. For an AI to be a partner in saving lives, its logic must be as visible as its results.
- The Explainability Gap: Deep Learning models often function as “black boxes,” where even the creators cannot fully trace the logic behind a specific prediction. In a high-stakes field like oncology or drug synthesis, “the computer said so” is an unacceptable rationale for a human trial.
- Regulatory Hurdles: Agencies like the FDA and EMA require rigorous “mechanistic proof.” They need to know why a molecule behaves a certain way to ensure long-term safety. AI must move toward Explainable AI (XAI) to meet these stringent evidentiary standards.
- Accountability and Liability: If an AI-designed drug fails unexpectedly, determining liability becomes a legal minefield. Transparency in the AI’s decision-making process is essential for establishing professional and legal accountability.
The Future: The Autonomous “Closed-Loop” Lab
The next frontier isn’t just a smarter algorithm; it is a physical laboratory that thinks and acts on its own, accelerating the pace of discovery from years to weeks.
- Human-AI Collaboration: The goal is not to replace the scientist, but to liberate them. By delegating the “brute force” experimentation to autonomous systems, human researchers can focus on high-level strategy, ethical oversight, and the complex nuances of patient care.
- The Iteration Engine: An autonomous lab creates a continuous feedback loop. The AI designs a digital blueprint, robotic arms synthesize the compound, and automated sensors test the results. The data is instantly fed back into the AI to refine the next “batch,” creating a 24/7 cycle of evolution.
- Scaling Scientific Intuition: These platforms don’t get tired or succumb to cognitive bias. They can test thousands of chemical combinations simultaneously—experimental scales that would be physically and mentally impossible for human teams to manage.
Conclusion
AI in drug development is moving past the hype phase and into the delivery phase. We are already seeing the first drugs designed entirely by AI entering human clinical trials.
By compressing timelines and reducing costs, AI doesn’t just mean higher profits for pharmaceutical companies; it means hope. It means that cures for rare diseases, previously considered unprofitable to research, may become viable. It means that when the next global health crisis emerges, our response time will be measured not in years, but in months. The collaboration between human biological intuition and artificial computational power is the new engine of medical breakthrough.
Frequently Asked Questions (FAQs)
1. How exactly does AI “speed up” the discovery of a new drug?
AI accelerates the process by replacing years of physical “trial-and-error” lab work with ultra-fast digital simulations. Traditionally, scientists had to physically test thousands of chemicals to see if they reacted with a disease.AI models like DrugCLIP can now screen 500 million molecules against human proteins in a single day—a feat that is roughly 10 million times faster than older computer methods. By predicting which molecules are likely to work and which will be toxic before they are ever made in a lab, AI narrows down the search from years to just months.
2. Can AI-designed drugs really be as safe as traditionally developed ones?
Yes, and potentially even safer. AI uses Predictive Toxicology to analyze “Absorption, Distribution, Metabolism, Excretion, and Toxicity” (ADMET) properties early in the process.
- Traditional safety: Often discovered late in expensive animal or human trials.
- AI safety: AI “digital twins” (computer simulations of human organs) predict adverse reactions with over 90% accuracy before a human ever touches the drug. This “fail-fast” approach ensures that only the safest candidates move forward.
3. How does AI help in the “Lock and Key” (Protein-Ligand) matching?
Finding a drug that fits a disease protein is like finding a key for a microscopic lock. The “AlphaFold moment” changed everything by using AI to predict the 3D shapes of nearly all 200 million known proteins. AI now uses Deep Contrastive Learning to turn these 3D shapes and potential drug molecules into digital vectors (mathematical maps). This allows the computer to “snap” them together virtually to find the perfect fit with staggering precision, identifying “keys” for diseases like cancer and autism that were previously considered “undruggable.”
4. Does AI mean we no longer need human clinical trials?
No, AI does not replace human trials, but it makes them much more efficient. AI is currently used to:
- Match Patients: Algorithms scan electronic health records to find ideal trial participants 10 times faster than manual screening.
- Predict Outcomes: AI analyzes real-time data from wearables and sensors to spot early signs that a drug is working (or causing a side effect).
- Synthetic Control Arms: In some cases, AI uses data from past patients to create a “digital placebo group,” reducing the total number of human volunteers needed for a study.
5. What are the biggest challenges currently facing AI in drug development?
Despite the speed, three major hurdles remain:
- The “Black Box” Problem: AI can find a cure, but it can’t always explain why it works. Regulators like the FDA require transparent reasoning to ensure safety.
- Data Quality: AI is only as good as the data it learns from. If the historical medical data is biased or incomplete, the AI’s “cures” will be flawed.
- The “Valley of Death”: While AI is amazing at the discovery phase (the first 1-3 years), the final human clinical trial stages still take several years because biological safety in a living human cannot yet be 100% simulated.
